Section: New Results
Meristem functioning and development
In axis 2 work focuses on the creation of a virtual meristem, at cell resolution, able to integrate the recent results in developmental biology and to simulate the feedback loops between physiology and growth. The approach is subdivided into several sub-areas of research.
Data acquisition and design of meristem models
-
Improvement of the MARS-ALT pipeline robustness Meristem, laser microscopy, image reconstruction, cell segmentation, automatic lineaging
Participants : Léo Guignard, Christophe Godin, Christophe Pradal, Grégoire Malandain [Morpheme, Inria] , Gaël Michelin [Morpheme, IPL Morphogenetics, Inria] , Guillaume Baty, Sophie Ribes [IBC, UM2] , Jan Traas [RDP, ENS] , Patrick Lemaire [CRBM, CNRS] , Yassin Refahi [RDP, ENS] .
This research theme is supported by a PhD FRM grant, Jan Traas's ERC, Inria ADT programme and the Morphogenetics Inria Project Lab.
The MARS-ALT (Multi-Angles Registration and Segmentation - Automatic Lineage Tracking) software pipeline automatically performs a segmentation at cell resolution from 3D or 2D voxel images where the membranes/walls are marked (by a die for example) and makes it possible to follow the lineage of these cells through time [5] . This year, a new version of this pipeline has been developed that uses informations redundancy across the movies and biological knowledge on the segmented organism to constrain and therefore improve the segmentation and the tracking. To test the new pipeline, we used different acquisition protocols and different organisms (floral and apical meristems and the early stages of development of a marine animal Phallusia mammillata). The segmentation is corrected a posteriori to deal with imaging artifacts due to uncertainties of acquisition. The image data set on which we develop the methods consists of :
-
Arabidopsis thaliana shoot apical meristem and primordia with around 6000 cells. The organ is captured from single angle every 4 hours during 2 or 3 days with a confocal microscope (Collaboration Sainsbury lab, Cambridge)
-
Arabidopsis thaliana flower meristems with around 2000 cells. The organ is also captured from single angle with a confocal microscope (Collaboration RDP Lyon and Sainsbury lab)
-
Phallusia mammillata embryos with from 32 cells to around 1000 cells. The organism is captured from four different angles every minute during 10 hours with a SPIM (Single Plane Illumination Microscope) (Collaboration CRBM Montpellier / EMBL Heidelberg). This work is developed in the context of the PhD work of Léo Guignard.
To our knowledgeIt is the first time that such high-resolution 4D digital tissues have been generated taking into account the cell shapes, opening the way to quantitative analysis of morphogenesis and tissue deformation at cell resolution.
-
-
Creating mesh representation of cellular structures (Guillaume Cerutti, Sohie Ribes, Christophe Godin)
Participants : Guillaume Cerutti, Sophie Ribes, Christophe Godin, Géraldine Brunoud [RDP, ENS] , Carlos Galvan-Ampudia [RDP, ENS] , Teva Vernoux [RDP, ENS] , Yassin Refahi [RDP, ENS, Sainsbury Lab] .
This research theme is supported the HFSP project Biosensors.
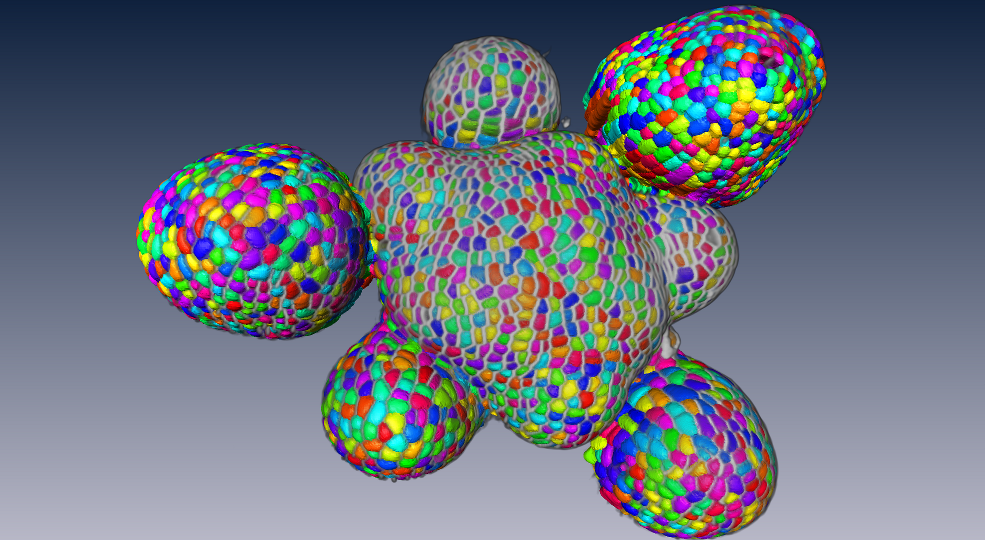
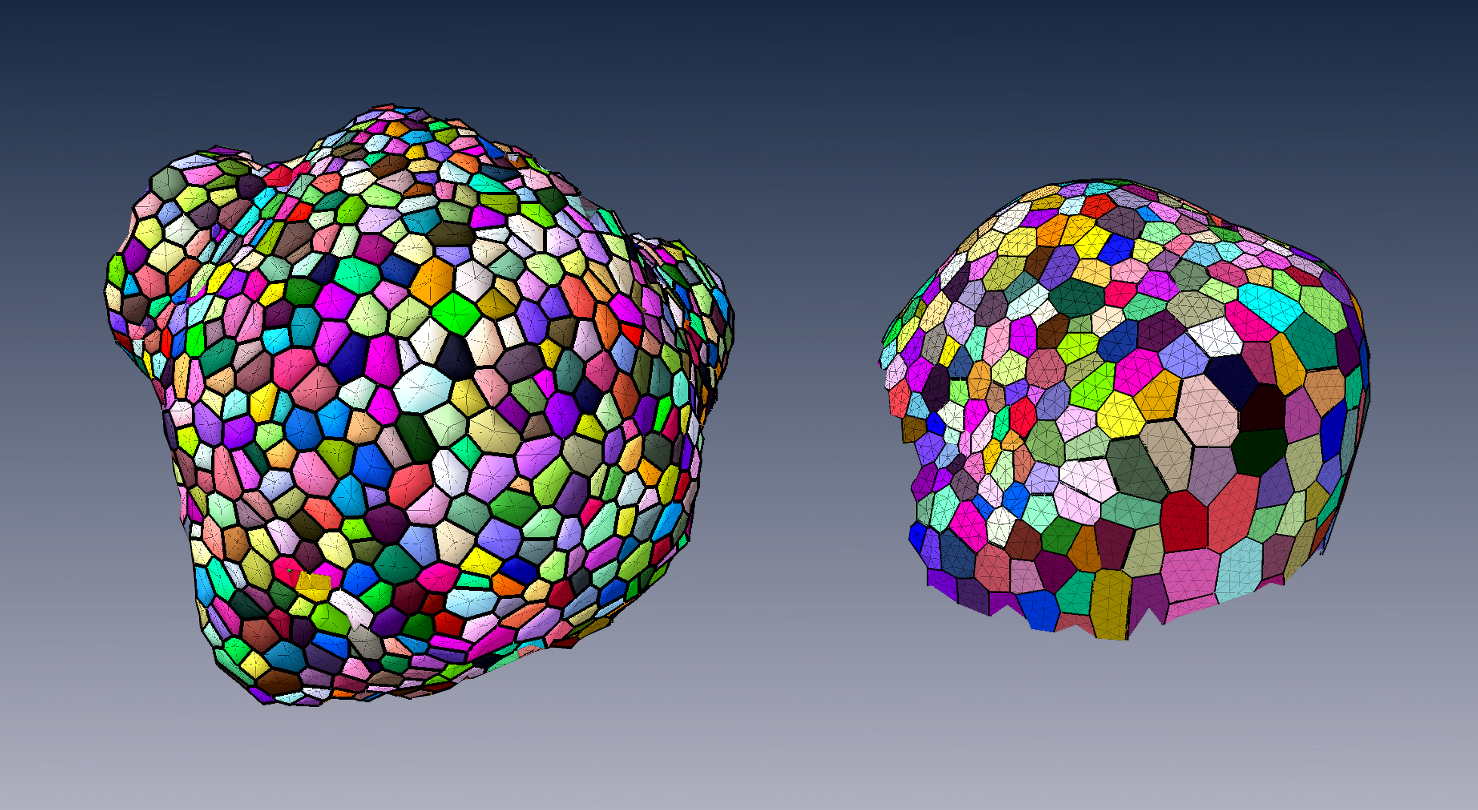
To produce a more efficient data structure accounting for the geometry of cellular tissues, we studied the problem of reconstructing a mesh representation of cells in a complex, multi-layered tissue structure, based either on membrane/wall images segmented using MARS or on nuclei images of shoot apical meristems. The construction of such mesh structures for plant tissues is currently a missing step in the existing image analysis pipelines. We developed a set of tools to build a triangular mesh surface representing the tissue in 3D, to evaluate the quality of the tissue reconstruction over objective aspects, to optimize a low-quality mesh simultaneously along several criteria, and to go towards a higher-scale representation pulling away from the cell resolution [31] . These methods are used in particular on nuclei images of shoot apical meristems of Arabidopsis thaliana to project hormonal information at cell-level on a continuous 3D tissue geometry. This work is carried out in the context of the post-doc of Guillaume Cerutti within the HFSP project BioSensors (Collaboration RDP Lyon).
These tools can produce light discrete representations of the cell interfaces that enables fast visualization, information projection, and quantitative analysis of the tissue, and give way to in silico physical and mechanical simulations on real-world data.
-
Design of 3D digital atlases of tissue development
Participants : Sophie Ribes, Yassin Refahi [RDP, ENS, Sainsbury Lab] , Guillaume Cerutti, Christophe Godin, Christophe Pradal, Christophe Pradal, Frédéric Boudon, Gregoire Malandain [RDP, ENS] , Gaël Michelin [RDP, ENS] , Guillaume Baty, Jan Traas [RDP, ENS] , Teva Vernoux [RDP, ENS] , Patrick Lemaire [CRBM, CNRS] , Françoise Monéger [RDP, ENS] .
This research theme is supported the Inria Project Lab Morphogenetics, the ADT Mars-Alt and the HFSP project Biosensors.
To organize the various genetic, physiological, physical, temporal and positional informations, we build a spatialized and dynamic database [56] . This database makes it possible to store all the collected information on a virtual 3D structure representing a typical organ. Each piece of information has to be located spatially and temporally in the database. Tools to visually retrieve and manipulate the information, quantitatively through space and time are being developed. For this, the 3D structure of a typical organ has been created at the different stages of development of the flower bud. This virtual structure contains spatial and temporal information on mean cell numbers, cell size, cell lineages, possible cell polarization (transporters, microtubules), and gene expression patterns. Such 3D digital atlas is mainly descriptive. However, like for classical databases, specific tools make it possible to explore the digital atlas according to main index keys, in particular spatial and temporal keys. Both a dedicated language and a 3D user interface are being designed to investigate and query the 3D virtual atlas. Current developments of this tool consist in using directly the segmented images produced from laser microscopy to build the atlas. To better represent the development of a biological population, a method to compute an "average" structure is investigated.
Shape analysis of meristems
Participants : Jonathan Legrand, Pierre Fernique, Frédéric Boudon, Yann Guédon, Christophe Godin, Pradeep Das [RDP, ENS] , Arezki Boudaoud [RDP, ENS] .
At cellular resolution, we studied the organization of cells in the meristems. The MARS-ALT pipeline provides rich spatio-temporal data sets for analyzing the development of meristems. A first step consisted of designing a dedicated graph for efficiently representing the spatial (adjacency between cells) and temporal (cell division) relationships between cells. Various variables can be attached either to the vertices (e.g. cell volume, inertia axes) or the edges (e.g. wall surface, distance between cell centroids). This graph may be augmented by new variables resulting from various spatial or temporal filtering (e.g. cell volumetric growth). Looking at homogeneous regions in the variable value space, cellular patterns can be identified. This work was developed in the context of the PhD of Jonathan Legrand with contributions of Pierre Fernique, another PhD student, that has been defended this year.
Considering the highly-structured nature of our data (time and space structuring) and the potential diversity and heterogeneity of possible cell descriptors, we developed two complementary approaches:
-
A first one that favours the spatial structuring: In this approach, the cell neighbourhood and the cell descriptors are jointly taken into account in a clustering approach whose objective is to identify a small number of clusters corresponding to well-defined cell identities. Once the cells have been labelled using the clustering algorithm, cell generation distributions are estimated on the basis of the labelled lineage trees.
-
A second one that favours the temporal structuring: In this approach, the data of interest are lineage forest and the only spatial structuring taken into account corresponds to siblings with respect to a given parent cell. In a first step, cell identities are inferred on the basis of the cell descriptors taking into account lineage relationships using hidden Markov tree models and the spatial regions that emerge from the cell identity labelling are then characterized. This second approach is supported by the fact that cell topology is only affected by division which makes highly relevant the local spatial information taken into account in this approach.
Mechanical model
Participants : Jean-Philippe Bernard, Olivier Ali, Christophe Godin, Benjamin Gilles, Frédéric Boudon, Ibrahim Cheddadi, Jan Traas [ENS-Lyon] , Olivier Hamant [ENS-Lyon] , Arezki Boudaoud [ENS-Lyon] .
This research theme is supported by the Inria Project Lab Morphogenetics and the Jan Traas's ERC.
The rigid cell walls that surround plant cells are responsible for the acquisition of organ shapes. These walls are submitted to stresses due to cell turgor pressure. Wall deformation is caused by the turgor forces in the cell walls. Wall synthesis is triggered by these wall deformations when some specific threshold is exceeded. The final shape of the tissue integrates mechanically all the local deformations of each cell.
To quantify this growth process at the level of a multicellular tissue, we developed a model of growth that integrates mechanical forces development at cellular resolution. In this model, walls are characterized by their mechanical properties, e.g. elasticity, extensibility and anisotropy. For this, we used a tensorial approach to describe both tissue deformation and stresses. Deformations were decomposed into elementary transformations that can be related to underlying biological processes. However, we showed that the observed deformations does not map directly local growth instructions given by genes and physiology in each cell. Instead, the growth is a two-stage process where genes are specifying how cell walls should yield to mechanical stresses. In this way, different regions in the tissue with different cell identities can have different growth properties. The final shape of the tissue results from the integration of all these mechanical properties and stresses at organ level under the growth force due to turgor pressure at tissue scale.
A paper describing the mechanical model and its application to model primorium formation in the shoot apical meristem will appear in PLoS Computational Biology in 2015. We used this framework to investigate the influence of a specific signalling cascade (the ABP1- Kat1 signalling pathway) and its putative mechanical consequences on primordium initiation [25] . A review of the different mechanical concepts underlying plant morphogenesis has been carried out in [12] .
In our first approach, the mechanical model rely on a finite element method (FEM) to describe the deformation of the tissue. In FEM, the tissue is represented by a mesh. The positions of the vertices at each time step are estimated from a linear system. If the tissue is big or if the mesh is fine, the linear system can be large and thus leads to computational overheads. An alternative way to classical FEM is to use a meshless method where the deformation of the tissue can be characterized by a linear combination of deformations of a finite and small set of frames. Because shape functions are no longer defined on each element but on the whole tissue, they have to be updated at each growth step by estimating a new rest configuration. With meshless method, the discretization of the system can be dynamically updated parsimoniously according to the precision required to model the emergence of shapes (PhD work of Jean-Philippe Bernard).
Gene regulatory networks: Design of a genetic model of inflorescence development.
Participants : Eugenio Azpeitia, Christophe Godin, François Parcy, Etienne Farcot.
This research theme is supported by the Inria Project Lab Morphogenetics.
Modeling gene activities within cells is of primary importance since cell identities correspond to stable combination of gene expression.
We studied the regulatory network that controls the flowering transition during morphogenesis. To overcome the network complexity and integrate this regulation during ontogenesis, we have developed a first model of the control of floral initiation by genes, and in particular the situation of cauliflower mutants, in which the repeatedly meristem fails in making a complete transition to the flower. Three different network models were done and validate. A first Boolean version, a second fuzzy logic and an ODEs models were studied. The models are able to correctly recover the gene steady states observed in the meristems during the flower transitions, the gene transitions and the mutant effects. Importantly, the model is able to explain the cauliflower mutants. This work couples models at different scales, since the gene regulatory network is used as a decision module in an L-system model of the inflorescence architecture. This mixed model has led us to make different hypotheses about gene interactions and hormonal regulation. First predictions about gene actors controling the passage to flower could be verified. Finally, some links between gene regulation and plant growth have been identified. These links can be experimentally tested which could lead to a first integrated picture of flower development could be reached in the context of Eugenio Azpeitia postdoc.
Model integration
Participants : Frédéric Boudon, Christophe Godin, Guillaume Baty, Jan Traas.
This research theme is supported by the Morphogenetics Inria Project Lab.
Our approach consists of building a programmable tissue which is able to accept different modeling components. This includes a central data structure representing the tissue in either 2-D or 3-D, which is able to grow in time, models of gene activity and regulation, models of signal exchange (physical and chemical) between cells and models of cell cycle (which includes cell division). For each modeling component, one or several approaches are investigated in depth, possibly at different temporal and spatial scales, using the data available from the partners (imaging, gene networks, and expression patterns). Approaches are compared and assessed on the same data. The objective of each submodel component will be to provide plugin components, corresponding to simplified versions of their models if necessary, that can be injected in the programmable tissue platform. This work is developed in collaboration with the RDP group at ENS-Lyon [58] and the CPIB group in Nottingham, UK [47] .
One key aspect of our approach is the development of a computer platform dedicated to programming virtual tissue development. This platform will be used to carry out integration of the different models developed in this research axis. The platform is based on OpenAlea. In the past year, progress has been made in defining a generic tissue data structure that could be used in this platform. Currently, robust geometric operations such as division are implemented and tested. Moreover, a redesign of the structure based on more elaborated formalisms such as combinatorial maps is investigated.